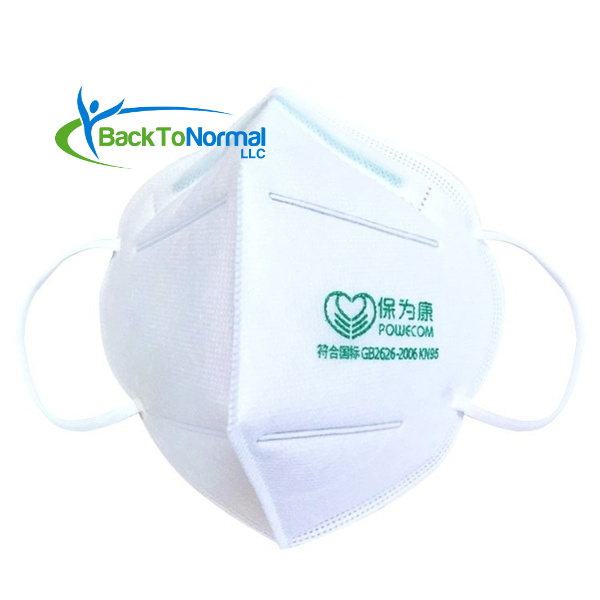
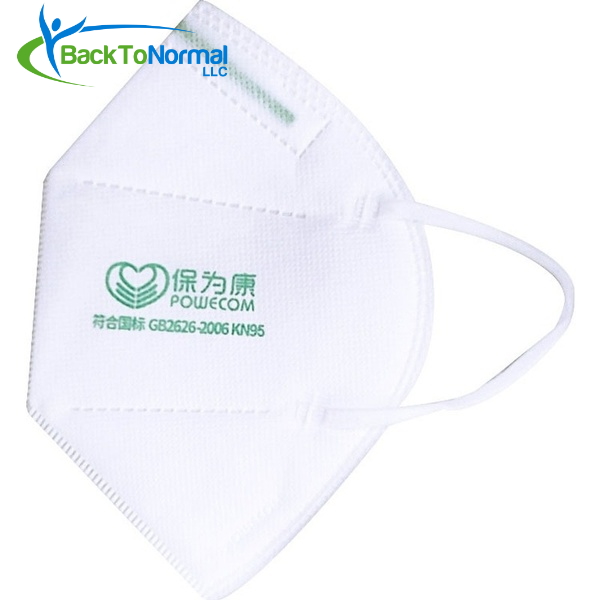
- On FDA’s whitelist (EUA) for medical use against COVID-19 exposure
- Ships same business day from Las Vegas, Nevada
- CDC recommends N95, KN95, FFP2 to protect from bioaerosols
- Four (4) layers of filtration
- Contains a metal strip that forms to your nose for a better fit
- Elastic ear straps hold masks in place comfortably
- Folds flat for easy storage
- #1 brand for medical and personal face masks in Asia
POWECOM KN95 Respirator (10 Masks / Pack)
Original price was: $45.00.$20.00Current price is: $20.00.
Out of stock
Out of stock
Healthcare professionals or medical institutions, contact us at sales@backtonormal.co or call 1-702-357-3350 for bulk orders.
Product Overview
POWECOM is the official provider of respirators for government use and crisis relief during viral epidemics in China.1
- SARS Severe acute respiratory syndrome – 2003
- H1N1 Influenza A (Swine Flu) – 2009
- H7N9 Asian Lineage Avian Influenza A (Bird Flu) – 2013, 2016, 2017
- African Swine Fever Virus (ASF) – 2019 to current
- COVID-19 Coronavirus (SARS-CoV-2) – 2019 to current
POWECOM KN95 respirators are certified to block out over 95% of non-oil particles of 0.25 microns in size, offering equivalent filtration as N95 respirators.*
- Particulate Filtration Efficiency (PFE) >95%, Bacterial Filtration Efficiency (BFE) >95%
- PM 2.5
- Materials: High efficiency melt-blown filter, soft non-woven fabric, thickened isolated cotton, and polypropylene
- Ten (10) respirator face masks per pack
- Color: White
- Brand: Powecom (Baoweikang)
- Manufacturer: Guangzhou Powecom Labor Insurance Supplies Co., Ltd. (DBA: Guangzhou Powecom Safety Goods Manufacturing Co. Ltd.)
Reference:
US Guidelines & Safety
Food & Drug Administration (FDA) issued an Emergency Use Authorization (EUA)1, specifically authorizing POWECOM KN95 respirator to be used in healthcare settings by healthcare personnel to prevent exposure to pathogenic biological airborne particulates from COVID-19.
The Center for Disease Control (CDC) recommends N95/KN95/FFP2 for use in high risk situations, especially by medical personnel during pandemics.2
See 3M Science’s technical bulletin3 for side-by-side comparisons of various country-specific standards used to grade the Filtering Facepiece Respirators (FFR).
Reference:
- Food & Drug Administration (FDA) Emergency Use Authorization order. See Appendix A.
https://www.fda.gov/media/136664/download - Center for Disease Control (CDC)
https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/crisis-alternate-strategies.html - 3M Science
https://multimedia.3m.com/mws/media/1791500O/comparison-ffp2-kn95-n95-filtering-facepiece-respirator-classes-tb.pdf
Each package is individually sanitized prior to packaging for mail.
Indications
If you feel you are at risk for exposure to COVID-19, please follow all CDC guidelines about prevention, testing, and treatment.
- Keep proper social distancing (6 feet),
- wash your hands thoroughly and often, and
- if you feel you may have been exposed, call your doctor immediately.
Returns or Exchanges
All sales are final due to hygiene and safety reasons. Please select carefully as we are unable to accept this product for an exchange, or a partial or full refund.
Disclaimer
*Effective protection under standard conditions when the wearer follow guidelines to properly fit the respirator around the nose, cheeks and chin, without the obstruction of facial hairs to ensure the wearer don’t breathe around the edges of the respirator.
All products are authenticated and manufacturers are vetted as current registrations holders with respective governing bodies.
Back To Normal LLC relies on the data from the FDA, CDC, 3M, and China Daily contained herein and makes no representations regarding the accuracy of such information. Back To Normal LLC will not be held liable for damages resulting from use or reliance upon this information.
Additional information
| Weight | 4 oz |
|---|---|
| Dimensions | 7 × 1.5 × 7 in |